Fenbendazole Purity Tests
✅
Sanare Lab LTD – Verified Testing and Quality for Fenbendazole Capsules
At Sanare Lab LTD, we place quality and integrity at the forefront of everything we produce. We specialize in standardized capsule formats featuring Fenbendazole, developed for customers who value consistency, testing, and full batch traceability.
📦 Production and Batch Control
Every bottle is produced in small controlled runs to ensure stable composition and accurate filling. Our capsules are manufactured under cleanroom conditions, sealed in protective containers, and labeled with batch numbers and best-before dates for reference and accountability.
Additionally, the compound we use is sourced from trusted facilities that follow validated quality standards. Each production lot is analyzed and monitored internally before being cleared for third-party testing.
🔬 Verified Testing Protocols
We partner with accredited laboratories in the United States to perform yearly third-party verification. These tests follow certified methods like:
-
HPLC (High-Performance Liquid Chromatography) — to confirm composition strength and detect inconsistencies
-
GC-MS (Gas Chromatography-Mass Spectrometry) — to rule out contamination or unintended compounds
-
Microbial testing — to verify that products are free from harmful biological agents
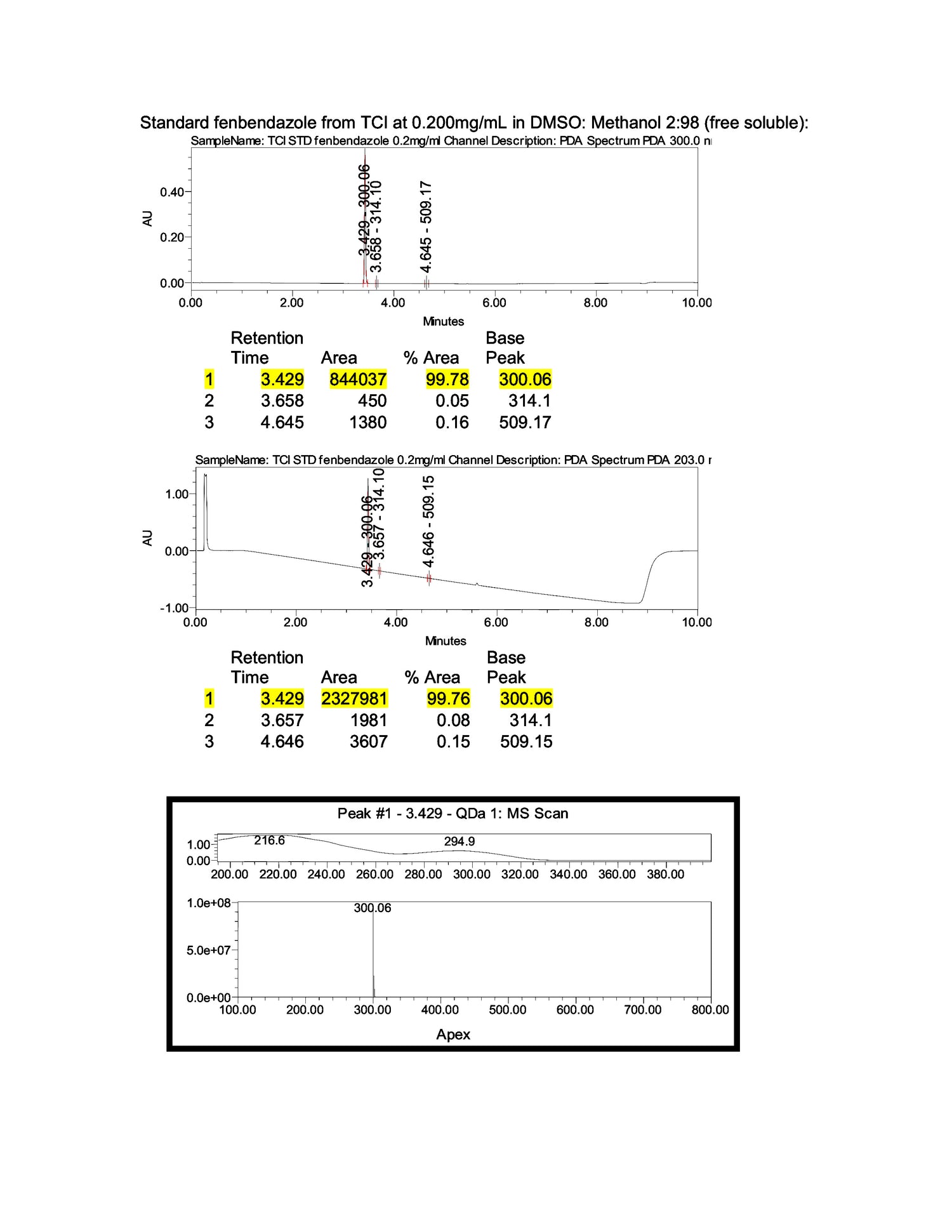
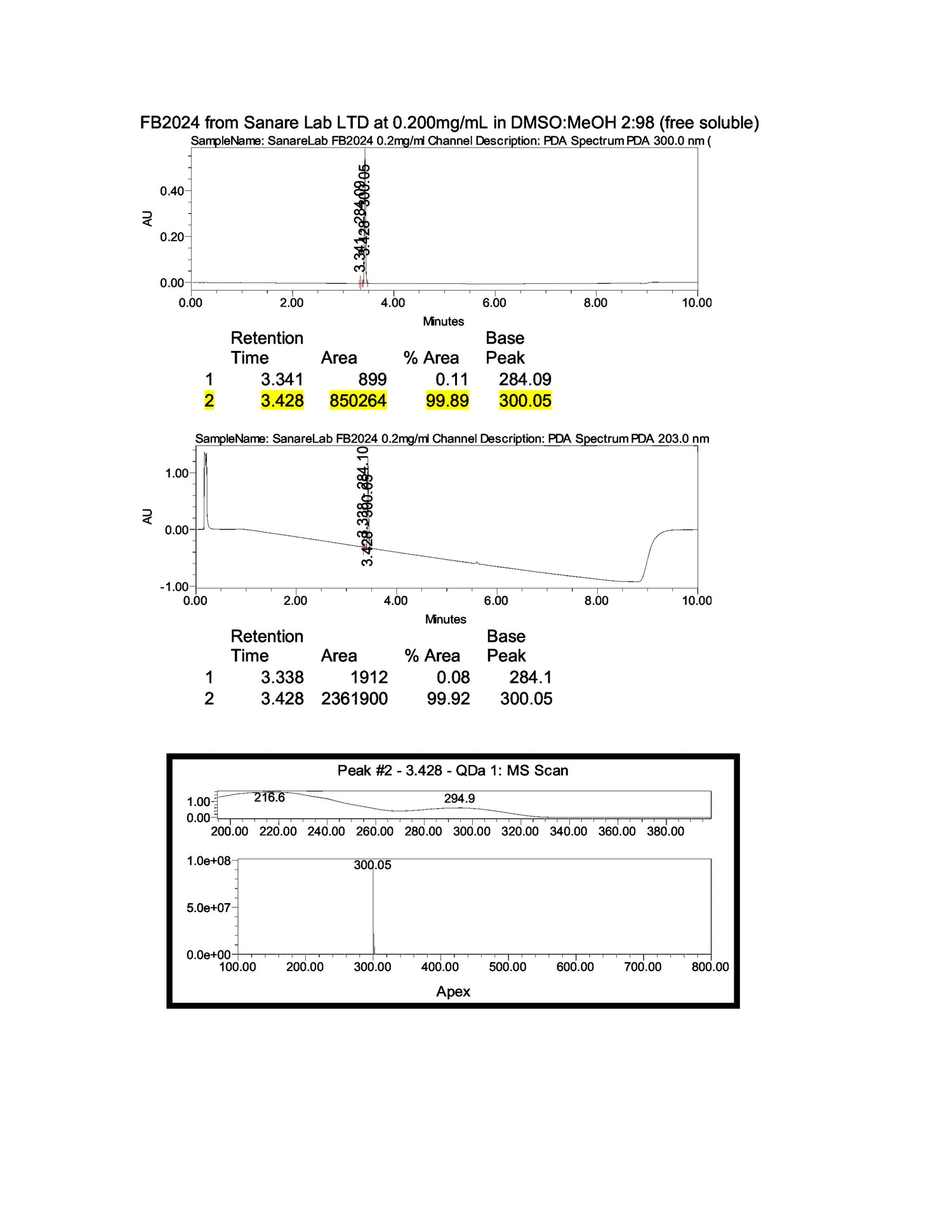
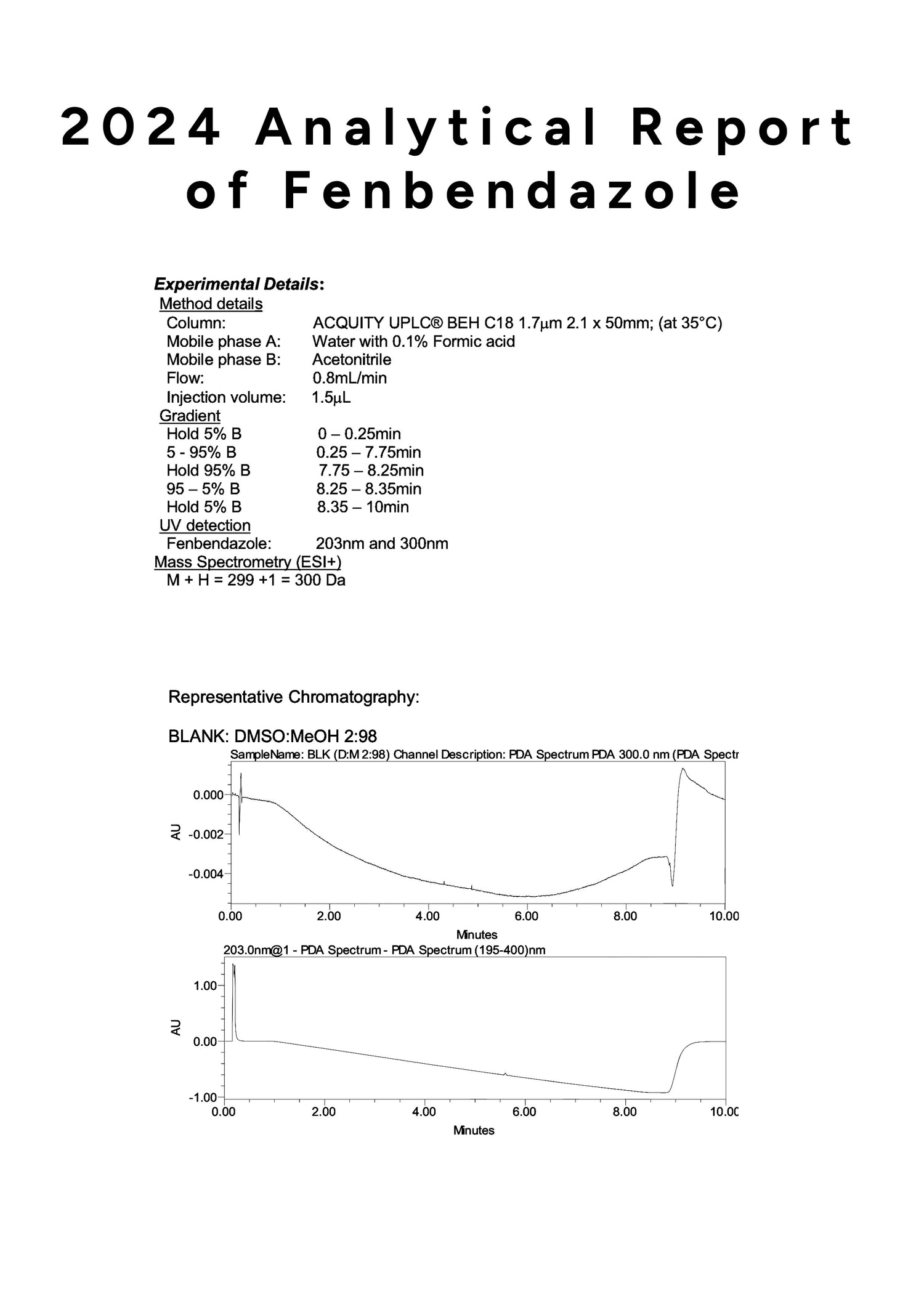
Furthermore, each test cycle is conducted under an assigned SOP and reviewed by lab directors. The latest results confirmed a consistent composition, with test outcomes exceeding 98.04% content accuracy, based on U.S. analytical standards.
📄 Certificates and Documentation
As a result of these evaluations, our products meet both internal expectations and external standards. We maintain clear records of all tests, and Certificates of Analysis (CoA) are available for each lot upon request.
This approach ensures that every customer has the opportunity to confirm the composition and quality of the Fenbendazole capsules they receive.
📈 Ongoing Quality Assurance
In addition to annual third-party testing, our internal quality team continuously monitors:
-
capsule weight and uniformity
-
moisture protection during packaging
-
label accuracy and compliance
Moreover, routine inspections and audits help confirm that each step of the production process aligns with good manufacturing practices (GMP).
🚚 Shipping and Availability
Sanare Lab currently ships exclusively within the United States. Most orders are fulfilled within 24–48 hours and include tracking via UPS or USPS. We offer bulk packaging options for larger customers, as well as ongoing delivery terms for recurring orders.
🔗 Additional Information
We believe in product clarity. That’s why each page of our website provides relevant details, including ingredient specifications and test coverage. For more on our lab procedures, visit our Fenbendazole Testing Overview, or learn About Sanare Lab.
2025 Testing:

Sanare Lab 2024 Testing
In 2024, laboratory tests confirmed that our Fenbendazole maintains a 99.9% purity level.